2023

2023
10 years PediatrOS™ FlexTack™
We are proud to celebrate 10 years of clinical use of our PediatrOS™ FlexTack™ Staples.
FlexTack™, the first anatomically shaped and flexible staple for growth guidance, has been successfully used since 2013 for the treatment of axial leg deformities using temporary hemi-epiphysiodesis in children and adolescents.
2022

01 / 2022
25 years of Merete
Merete celebrates its company anniversary: Merete GmbH has been developing medical devices since 1996 – and we’ve been passionate about customer-oriented solutions since the very beginning. Throughout the past 25 years, we’ve remained a reliable partner in navigating everyday clinical practice, reacting to market needs with speed and agility.
Merete products have proven themselves worldwide and are now used in over 40 countries. Thanks to an excellent team of engineers, quality managers, product specialists and sales employees working in close cooperation with renowned international partners, our products have established themselves as indispensable standards in medical technology over the past 25 years.
2020

01 / 2020
BioBall® goes ...
… Switzerland and France. Merete has taken over direct sales for the tried-and-tested BioBall® Adapter System in Switzerland. We have also found a strong distribution partner for the French market in Lima France. Despite the coronavirus crisis, the tried-and-tested BioBall® Adapter System is now continuing to expand worldwide.
2019

01 / 2019
Successful extension of BioBall® approval
The extension of the BioBall®’s CE market approval through 2024 represents a crucial milestone in the recertification phase.
Merete has thus proven once again that it is a long-term partner in the field of endoprosthetics.
2019

2019
New addition to management
Besides successfully extending our CE approval, another major recent event was bringing Dr. Ing. Marc Kneissler on board to bolster our management team.
2017

2017
20 years of Merete
For 20 years now, Merete has been synonymous with German innovation and proven solutions on the bone surgery medical devices market.
Merete remains fully committed to patient well-being, continually developing new products and service-oriented concepts in close cooperation with surgeons and hospital managers. Read about the very positive reception to the BioBall® System among long-term users in Merete&Friends Magazine.
2015
2015
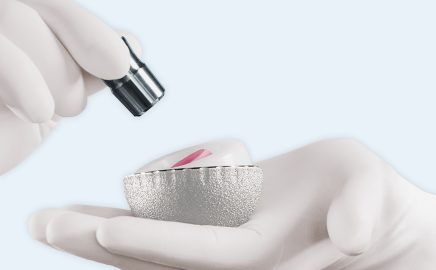
Dual mobility goes BioBall®
Expansion of the tried-and-tested BioBall® Adapter System continues with the DualMobility BioBall® MaxiMotion™.
The modular solution for preventing luxation in primary care and revision is implanted for the first time in 2015.
2015

2015
Alexia Anapliotis, CEO of Merete GmbH
Alexia Anapliotis, daughter of Merete founder Emmanuel Anapliotis, takes over as CEO of Merete. She has been actively involved in the company’s development since its founding.
2013

2013
First successful operation with PediatrOS™ FlexTack™ / RigidTack™
PediatrOS™ FlexTack™ and PediatrOS™ RigidTack™ staples are implants designed for growth steering in children and adolescents via temporary hemi-epiphysiodesis.
The first implant operation was successfully completed at the University Hospital of Munster in 2013. Both staple implants received FDA approval in December of 2015.
2013
2013
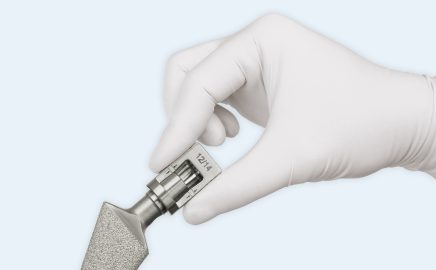
BioBall® AdapterSelector™ approval
The tried-and-tested BioBall® Adapter System has been expanded to include the BioBall® AdapterSelector™, which offers greater certainty when evaluating the taper on the existing stem during hip revision procedures.
The only instrument for taper checking approved worldwide is protected by patent law in both Europe and the USA.
2013

2013
10 years of MetaFix™
The MetaFix™ product family has been expanding continuously ever since the MetaFix™ I fixed-angle plate for correcting Hallux valgus was first introduced in 2003.
By 2017, this product family also included eight additional surgical bone plates specially developed for fixed-angle treatment of various conditions.
The FDA approved the MetaFix™ BLP for the US market in 2005. Additional approvals followed in the years 2006-2014, resulting in Merete achieving a strong position on the US market with its FootFamily product group.
2006

2006
Welcome to the United States of America!
Merete USA founded. In addition to the BioBall® system, its podiatric surgery products and the OsteoBridge™ system have also enjoyed widespread recognition in the USA.
Since entering the US market, Merete has already received 25 FDA approvals.
2003
2003
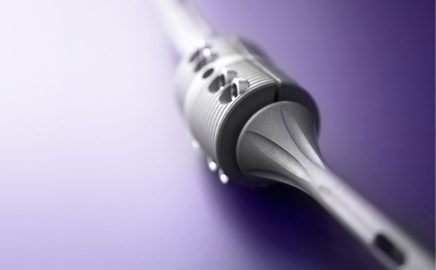
Market launch of the OsteoBridge™ System
The OsteoBridge™ system for diaphyseal treatment of tubular bones following fractures or tumour resections received its CE mark in 2003. The OsteoBridge™ System product group has been constantly expanding since OsteoBridge™ implants were first brought onto the market (with FDA approval following in 2006). Users now have access to two modular solutions for bridging bone defects and providing intramedullary treatment of complex, periprosthetic fractures:
OsteoBridge™ Knee Arthrodesis
Stiffening of the knee joint for temporary or permanent bridging between femur and tibia.
OsteoBridge™ IntraDocking™
Intramedullary treatment of periprosthetic and interprosthetic fractures, available only as a custom-made device.
2002

2002
Merete offers solutions for Hallux valgus
Successful entry into the podiatric surgery market, including with the MetaFix™ I Foot Plate; development and production of additional fixed-angle plates and screws for hallux valgus treatment.
1999

1999
BioBall® System approved as a “modular head system for hip revision surgery”
Since its approval, the BioBall® Adapter has established itself as an indispensable system; it is now considered the gold standard in hip surgery when treating in situ stems.
It received FDA approval in 2013. Today, users in more than 33 countries trust the proven modular head system in revision operations.
1996

1996
Founding
Merete was founded in 1996 by Emmanuel Anapliotis († 11 September 2022). With over 45 years of experience in the development and design of medical devices, he was a pioneer of the modern orthopaedic implant industry in Germany.
His company, Mecron Med. Produkte GmbH Berlin, produced a number of innovative products during the 1970s and 80s, including duo-head bipolar hip endoprostheses, spherical screw cups, MecroSet modular resection prostheses, Mecron cannulated screws, and Mecron knee braces. Emmanuel Anapliotis also drove innovation in the Merete Group for more than two decades.
Merete GmbH was founded in 2016. The Berlin-based company is responsible for research and development, regulatory affairs, sales and marketing.